Easy View form is a format for viewing the AER case details in a user friendly collapsed view.
In ESM, you can view the ICSRs (for both export and import cases) in Easy View format on the Import Section page and Export Section page and MDRs (for only export cases) in Easy View format on the Export Section page.
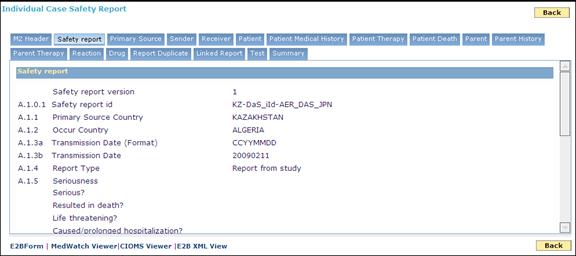
A sample of the ICSR file in Easy (collapsed ) view:

Easy View – Tabbed view
In the Easy View, you can view the details of an ICSR in the following sections:
M2 Header:In this section, you can view the details such as message type, message number, and sender ID and receiver ID.
Safety Report: In this section, you can view the safety report details such as safety report ID, primary source country, Occur country, received and receipt dates, authority number and company number.
Primary Source: In this section, you can view the reporter details and study details.
Sender: In this section, you can view the sender details.
Receiver: In this section, you can view the receiver details.
Patient: In this section, you can view the patient's personal details.
Patient Medical History: In this section, you can view the patient's medical history.
Patient Therapy: In this section, you can view the details of the drug given to the patient and the drug reaction details.
Patient Death: In this section, you can view the patient death and autopsy details if the consumption of the drug is resulted in death.
Parent: In this section, you can view the parents details of the patient.
Parent History: In this section, you can view the medical history of patient's parents.
Parent Therapy: In this section, you can view the parents' medical treatment details
Reaction: In this section, you can view the drug reaction details of the drug that is given to the patient.
Drug: In this section, you can view the details of the drug given to the patient.
Report Duplicate: In this section, you can view the duplicate details of the safety report if the selected report is a duplicate of the existing case.
Linked Report: In this section, you can view the details of the existing safety report that is linked to the current safety report.
Test: In this section, you can view the details of the medical tests that the patient has undergone.
Summary: In this section, you can view the details such as, case narrative (in local language), reporter's comment, sender's diagnosis and sender's comment.
In the Easy View, you can view the details of an MDR in the following sections:
Message Header: In this section, you can view the details such as sender ID, receiver ID message date, batch ID and number of MDRs associated with the message.
A. Patient: In this section, you can view the patient's personal details.
B. Adverse Event: In this section, you can view the adverse event information specific to a medical device.
D. Suspected Medical Device: In this section, you can view the information related to the suspected medical device associated with current MDR.
E. Initial Report: In this section, you can view the reporter details.
F. User Facility/Importer: On this tab, you can view the user facility details and importer details.
G. All Manufacturers: In this section, you can view the contact information, source of MDR, type of MDR and manufacturer report number.
H. Device Manufacturer Only: In this section, you can view the reportable event type, information of the manufacturer who evaluated the reported device, device manufactured date, evaluation codes, remedial action taken after the adverse event and information on device usage.
Expand each section to view the respective details or click Expand All to view the details of all the sections.
Note: In the E2B Easy View, you cannot enter or modify the data.
The system supports submitting of Korean R3 case and the corresponding Easy view and XML view display the Korean R3 tags in Export and Import Listing screen.