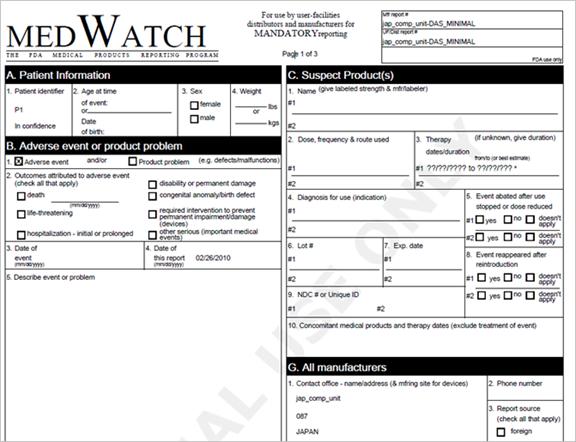
MEDWatch form is the Food and Drug Administration’s (FDA) reporting system format for viewing the adverse event information.
In ESM, you can view and print the cases in MEDWatch format on the Import Section page, Export Section page and Reports: Transaction page.
A sample of the ICSR file in MEDWatch Report View:

FDA Report View