
This section explains about the various aspects of reviewing a case before submitting the case to the recipient or an authority.
On the Export Section page > E2B (R2), E2B (R3), eMDR (R2), eVAER(R3) or MIR tab, click the AER Number link. You can view the AER details for a case in the AER Details window.

AER Details Window
AER Details - Field Description Table
Field Name |
Description |
Message Number |
The message number associated to the case. A message number is a unique identification number generated for the AER cases, by the application that can contain multiple safety report records or medical device report records. The Message number is a reference for the corresponding AER number and the safety report or device report. ESM Server generates the message number for each ICSR/ICSRACK and eMDR/eMDRACK files in the following format: <Account Name><Running Oracle Sequence> <messagenumb>PFZ0000000001234</messagenumb> For Example: If 'PFZ' is the account name for an E2BService and '0000000001234' is the Oracle sequence for the current operation, then the message number generated is 'PFZ0000000001234'. |
Number of AERs |
The total number of AERs (Safety reports or device reports) associated with the message number. |
AER Number (Ver.Loc Ver) |
The adverse event reaction number of the case. Ver denotes the version of the case and Loc Ver denotes the local version of the case. For example: IL-AER-097 (0.0). AER number is automatically generated during data entry in ARISg. The number uniquely identifies an AER in ARISg, and is automatically generated or manually entered based on the option set by the company, while setting the installation parameters in ARISg Administration section. |
Ver |
The latest version number of the case. |
Product Name |
The name of the medicine, medicinal product or medical device due to which the adverse event reaction occurred. |
Event |
The reported term for the adverse event of the case. |
Seriousness |
The severity of the case. |
Latest Received Date |
The date on which the safety report is received from ARISg. |
You can view the information of the receiver who received the case.
In the Export
Case page > E2B
(R2), E2B (R3), eMDR (R2), eVAER(R3)
or MIR tab, click![]() .
.
The Receiver’s Detail page appears.
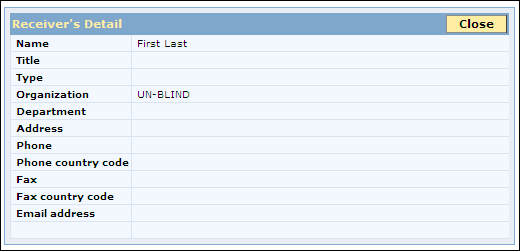
Receivers Details
To return to the Export Section page, click Close.
Receiver’s Field Details Table
Field |
Description |
Name |
The name of the recipient who receives the case. The recipient can be a regulatory authority or a business partner. |
Title |
The title of the receiver who receives the case. |
Type |
The profession of the receiver. The receiver can be any one of the following types:
|
Organization |
The name of the organization to which the receiver belongs. Note: System will display "Unit Name (Japanese)" for Japanese cases in export listing. |
Department |
The name of the department in the organization, to which the receiver belongs. |
Address |
The address of the receiver. |
Phone |
The phone number of the receiver. |
Phone Country Code |
The country code of the phone number. |
Fax |
The fax number of the receiver. |
Fax Country Code |
The country code for the fax number of the receiver. |
Email Address |
The email address of the receiver. |
To return to the Export Section page, click Close.
Application displays the following page when case is not available for export.

Export - Error Message Page
Note: Application allows only a single case export for MHLW distribution.