|
Field Name |
Description |
|
Date Range From-To |
The date range for which the report is generated. Application considers the date format mentioned in the user preferences. |
|
Development International Birth Date (DIBD) |
Date of first approval (or authorisation) for conducting an interventional clinical trial in any country. The application considers this date for calculating ’From date’ for Summary tabulation. If this date is not entered, then the application considers the DIBD from CPD for the selected product. If DIBD is greater than the ’From date’ entered in selection criteria, then ’From date’ is considered for calculating the counts in cumulative summary tabulation. |
|
When informed Authority is not considered |
Indicates the selection of the parameter Include cases without informed authority information in the Admin application. |
|
Date type |
The type of date considered for date range. Date type is available only when the parameter Include cases without informed authority information is not selected. Select the date type from the drop-down list. The following options are available:
|
|
When informed Authority is considered |
Indicates the selection of the parameter Include cases without informed authority information in the Admin application |
|
Alert date type |
Indicates the type of report for Authority. Select the Alert date from the drop-down list. The following options are available:
|
|
Alert/Non Alert Date type |
Select the Non-alert date from the drop-down list. The following options are available:
Alert/Non-alert date types are enabled only if the parameter Include cases without informed authority information is not selected. |
|
Other Info. |
|
|
Authority |
The approval authority of the report.
Select appropriate approval authority from the drop-down list. By default the option is Null. |
|
Report Language |
The language in which the report is printed. By default the option is Null |
|
Labeling Country |
Indicates the country for which the labeling of the drug is selected. If nothing is selected, then by default the application considers labeling country as International. By default the option is Null. |
|
Listedness from |
Indicates if the labelling is considered from the case or from the Company Product Dictionary. The available options are:
|
|
Include solicited cases |
Indicates the inclusion of solicited cases in the report. Select the checkbox to consider solicited source in the DSUR. This option is not selected by default. |
|
Select any one of the following. Select either Product or Study. |
|
|
Product |
|
|
Preferred Product Description |
Indicates the product description from the CPD lookup. By default the option is Null. |
|
Use PPD Code |
Select the check box to generate the DSUR report for a specific trade name along with its PPD code. Note:The product’s PPD code is constant and does not change when its trade name changes. |
|
Include associated products in the LL |
When the search criteria is based on Product (PPD) and/or Study Protocol, selecting this checkbox prints only those cases in the Line Listing (LL) where the products or comparator product associated with the respective case has Assess Relationship set to Yes. • If the search is based on Product (PPD), the application considers all those Cases where the products or comparator product associated with the respective case has Assess Relationship set to Yes. • If the search is based on Study Protocol, the application search for those cases matching the protocol selection criteria and considers only those cases for printing in the LL report where the products or comparator product associated with the respective case has Assess Relationship set to Yes. The cases that are mapped to Source (A.1.4) as “Observational Study” (Standard code 28 in codelist) are excluded and printed under exception section in the DSUR Report, even in the scenario where the Study Protocol criteria are provided. NOTE: The validation message appears in the following scenarios on clicking Generate in the DSUR screen: • Product or Study should be entered – This message appears when the “Include associated products in the LL” parameter is not selected, and the Study Protocol parameters (Project no./Protocol no./EudraCT no.) and Preferred Product Description is blank. • Product should be entered - This message appears when the “Include associated products in the LL” parameter is selected, and the Study Protocol parameters (Project no./Protocol no./EudraCT no.) and Preferred Product Description is blank. • Either Product or Study should be entered - This message appears when the “Include associated products in the LL” parameter is selected, and any one of the Study Protocol parameters (Project no./Protocol no./EudraCT no.) is not selected and Preferred Product Description is blank. |
|
Study info |
|
|
EudraCT No. |
A unique identification number of a clinical trail in the European Union. Indicates the EudraCT No. of the study case for which DSUR is generated. By default the option is Null. |
|
Protocol no. |
Study number of a case. To enter the Protocol number:
By default the option is Null. For more information on Lookups, refer Data Entry Features guide. |
|
Project no. |
Enter the Project number. By default the option is Null. |
|
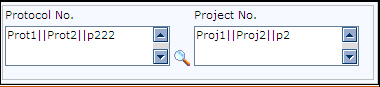
When you select multiple Protocol numbers in the Protocol Lookup screen, all the protocol numbers and project numbers you have selected are displayed in the Protocol No. and Project No. fields with a separator (||) between each of the protocol numbers and project numbers. For example: If you select Prot1, Prot2 and Prot3 in the lookup, then these protocol numbers and project numbers are displayed as shown in the following figure:
|
|
|
Group By- Check the options to group the cases in Line listing and Summary Tabulation sections of DSUR. The grouping of cases is in following order:
|
|
|
Protocol number |
Select this option to group the cases by Protocol number in Line listing and Summary Tabulation sections of DSUR. |
|
Initial/Follow-up |
Indicates the printing of Initial and Follow-up cases. |
|
Print option |
Indicates the grouping of Line listing and Summary Tabulation. This option is available only when Initial/ Follow-up grouping is selected. By default Both is selected. |
|
SOC |
SOC is a group of adverse reaction Preferred terms pertaining to the same system-organ. Select the checkbox to group the Line listing cases. The group by SOC is applicable only for line-listing cases. |
|
Others |
Select either labeling or indication to group the case. The case gets listed under UNKNOWN, if there is no data available for the group by option set. For example, if group by indication is selected and if indication is not available for a case, then the case gets listed under UNKNOWN. |